2021 Volume 46 Issue 11 Pages 509-514
2021 Volume 46 Issue 11 Pages 509-514
Dihydropyrazines (DHPs) are one of glycation products that are non-enzymatically generated in vivo and in food. We had previously revealed that 3-hydro-2,2,5,6-tetramethylpyrazine (DHP-3), a methyl-substituted DHP, elicited redox imbalance and cytotoxicity in cultured cells. However, the molecular mechanisms underlying DHP-3-induced cytotoxicity remain unclear. To address this issue, we examined the involvement of the receptor for advanced glycation end products (RAGE) in DHP-3-induced cytotoxicity. To evaluate the role of RAGE, we prepared HeLa cells that constitutively expressed RAGE and its deletion mutant, which lacks the cytoplasmic domain (RAGEΔcyto), using an episomal vector. After transfection with the vector, cells were selected following incubation with multiple concentrations of hygromycin to remove non-transfected cells. The expression of RAGE and RAGEΔcyto in the cells was confirmed by immunoblotting. RAGE and RAGEΔcyto were apparently expressed in transfected cells; however, there were no significant differences in DHP-3-induced cytotoxicity between these cells and mock vector-transfected cells. These results suggested that DHP-3 elicits cytotoxicity in a RAGE-independent manner.
Glycation products are non-enzymatically generated through the Maillard reaction and accumulate in the body with aging. They are suggested to be predisposing factors for various inflammatory disorders, including cancer, diabetes, and neurodegenerative diseases (Tessier, 2010; Brownlee, 1995; Vicente Miranda et al., 2016). Therefore, clarification of the biological effects and molecular mechanisms induced by glycation products is important for the prevention and treatment of these disorders. Dihydropyrazines (DHPs) are intermediates of glycation products whose skeletons are generated by dimerization of either D-glucosamine (Kashige et al., 1995) or 5-aminolevulinic acid (Teixeira et al., 2001). Many pyrazine derivatives, which are potential metabolites of DHPs, have been detected in foods (Joo and Ho, 1997; Maga, 1982) and human urine (Zlatkis et al., 1973). Thus, DHPs are predicted to be ubiquitously present in the body and the environment. So far, we have investigated the biological effects of DHPs by focusing on methyl-substituted DHPs. Methyl-substituted DHPs produce several kinds of radicals (Yamaguchi et al., 2012), exhibit DNA strand-cleaving activity (Kashige et al., 2000; Yamaguchi et al., 1996), induce growth inhibition and mutagenesis, and inactivate glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in E. coli (Takechi et al., 2010, 2004). In addition, we revealed that 3-hydro-2,2,5,6-tetramethylpyrazine (DHP-3, Fig. 1) has several cellular effects, including cytotoxicity through redox imbalance (Ishida et al., 2012, 2014; Takechi et al., 2011, 2015) and the repression of Toll-like receptor 4 pathway in lipopolysaccharide (LPS)-pretreated cells (Esaki et al., 2020). Despite these interesting biological effects, the molecular mechanisms underlying these DHP-3-induced effects remain unclear.
Chemical structure of 3-hydro-2,2,5,6-tetramethylpyrazine (DHP-3).
In this study, we hypothesized that the receptor for advanced glycation end products (RAGE) mediates DHP-3-induced cytotoxicity. RAGE is a type I transmembrane protein that functions as a pattern recognition receptor (Hudson and Lippman, 2018). RAGE was originally defined as a receptor for advanced glycation end products (AGEs); however, it is now known that the receptor also recognizes many other molecules, such as high mobility group 1 protein (Hori et al., 1995), S100 calgranulins (Hofmann et al., 1999), amyloid-β peptide (Yan et al., 1996), and LPS (Yamamoto et al., 2011). Activated RAGE signaling elicits oxidative stress by generating reactive oxygen species (Wang et al., 2016; Wautier et al., 2001; Yan et al., 1994) and upregulates its expression level through positive feedback (Bierhaus et al., 2005). Based on these features, the involvement of RAGE in DHP-3-induced cytotoxicity is sufficiently postulated. To examine this hypothesis, we constructed HeLa cells expressing RAGE or its deletion mutant lacking the cytoplasmic domain (RAGEΔcyto) and observed the effects of DHP-3 on these cells.
DHP-3 was synthesized according to a previously described method (Yamaguchi et al., 1996). Synthetic oligonucleotides were purchased from Fasmac (Kanagawa, Japan). Restriction enzymes were obtained from Takara Bio (Shiga, Japan). All other reagents were of the highest commercially available grade.
Subcloning of RAGE and its mutantThe open reading frame (ORF) of RAGE was amplified by polymerase chain reaction (PCR) with the pcDNA3-RAGE vector (Huttunen et al., 1999) that was provided by Henri Huttunen (Addgene plasmid #71435; http://n2t.net/addgene:71435; RRID: Addgene_71435). KOD One PCR Master Mix (TOYOBO, Osaka, Japan) was utilized for the PCR reactions, and the primers used were as follows: forward, 5’-cgg gcc ccc cct cga acc atg gct gcc gga aca-3’; reverse, 5’-tct aga gtc gcg gcc tca agg ccc tcc agt act-3’. The RAGE mutant (RAGEΔcyto), which lacks the C-terminal cytoplasmic domain of RAGE, was constructed as illustrated in Fig. 2A. It has been established that this mutant is unable to transduce any inflammatory signals to the downstream pathway, although its ligand-binding ability is retained (Huttunen et al., 1999). Therefore, we expected RAGEΔcyto to function as a negative control if DHP-3 induced cytotoxicity through RAGE. The ORF of RAGEΔcyto was also generated using the same procedure and the following primers: forward, the same as wild-type RAGE; Reverse, 5’-tct aga gtc gcg gcc tca cca caa gat gac ccc-3’. The amplified ORFs were subcloned into NotI/XhoI-digested pEBMulti-Hyg vector (FUJIFILM Wako Pure Chemical, Osaka, Japan) using the In-Fusion HD Cloning Kit (Takara Bio). The nucleotide sequences of the constructs were confirmed by Sanger sequencing (GENEWIZ Japan, Tokyo, Japan).
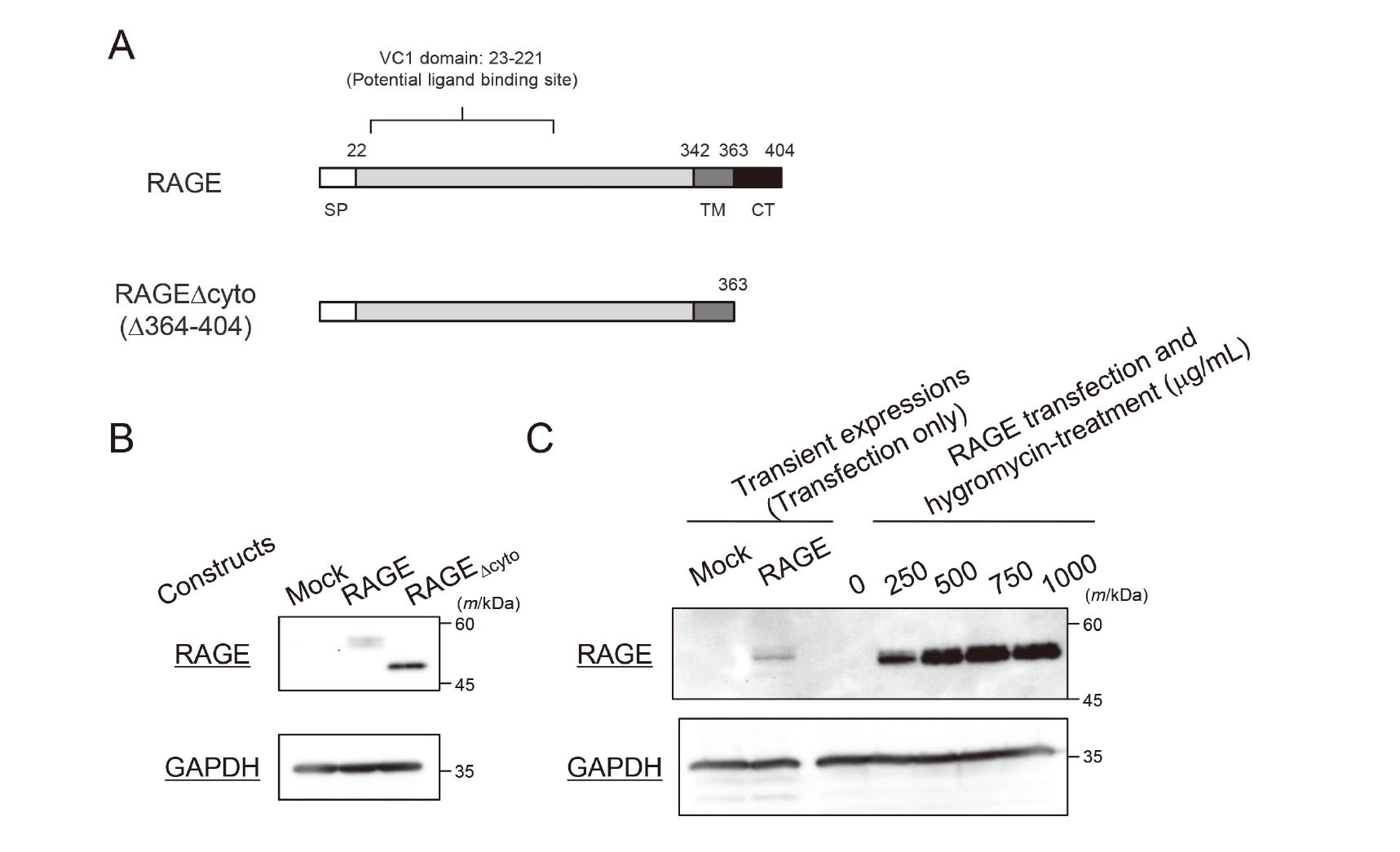
Construction of HeLa cells constitutively expressing RAGE or its deletion mutant (RAGEΔcyto). (A) Schematic sequences of RAGE and RAGEΔcyto. The numbers represent the residue position of RAGE from the N-terminus. SP, signal peptide; TM, trans-membrane region; CT, cytoplasmic domain (B) Immunoblotting to confirm transient expression of RAGE and its mutant in HeLa cells. The recombinant expression vectors were transfected into HeLa cells, and the cells were collected 48 hr after transfection. HeLa cell lysates (20 μg) were analyzed. GAPDH was also detected as a loading control. (C) Determination of the hygromycin concentration to select RAGE- and RAGEΔcyto-expressing HeLa cells. The left two samples were prepared 48 hr after transfection (transient expression), and the others were further cultured in the presence of hygromycin for another week. Cell lysates (20 μg) were analyzed by immunoblotting.
HeLa cells (JCRB9004) were obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan) and cultured in low glucose Dulbecco’s modified Eagle’s medium (DMEM) (FUJIFILM Wako Pure Chemical, Cat#041-29775) supplemented with 10% fetal bovine serum (FBS). Cells were routinely maintained in a 10 cm dish and seeded the day before transfection. HilyMax transfection reagent (Dojin Molecular Technologies, Kumamoto, Japan) and expression vectors (RAGE-pEBMulti-Hyg vector and RAGEΔcyto-pEBMulti-Hyg vector) or mock vectors (empty pEBMulti-Hyg vector) were mixed in Opti-MEM™ I Reduced Serum Medium (Thermo Fisher Scientific, Waltham, MA, USA). After incubation for 15 min at room temperature, the mixture was added to the cells. The medium was replaced at 4 hr after transfection, and the cells were collected 48 hr after transfection. Transient expression of RAGE and RAGEΔcyto were confirmed (Fig. 2B). The episomal vector we utilized can be duplicated during cell division and transmitted in daughter cells. Thus, we selected the HeLa cells that constitutively expressed RAGE or RAGEΔcyto with hygromycin treatment following transient expression. For the selection, the cells were further cultured in the presence of hygromycin for a week. During selection, exposure to various hygromycin concentrations, from 0 to 1,000 µg/mL, was conducted to determine the optimum concentration for the reliable selection of transfected cells. As a result, 500 µg/mL of hygromycin was used (Fig. 2C).
Preparation of cell lysates and immunoblottingThe expression of RAGE and RAGEΔcyto in transfected HeLa cells was confirmed by immunoblotting. Cells were collected in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, and 10% glycerol, and then sonicated with an AS38A ultrasonic cleaner (AS ONE, Osaka, Japan). Protein concentrations were determined using Quick Start Bradford 1 × Dye Reagent (Bio-Rad Laboratories, Hercules, CA, USA) with bovine serum albumin as a standard. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto a polyvinylidene difluoride membrane. The membrane was washed with Tris-buffered saline (pH 7.4) containing 0.1% Tween 20 (TBS-T). After blocking with 5% skim milk in TBS-T, the membrane was incubated with primary antibody diluted 2,000-fold with 5% skim milk in TBS-T. The primary antibodies used were as follows: mouse anti-RAGE (sc-74473; Santa Cruz Biotechnologies, Dallas, TX, USA), rabbit anti-NF-κB p65 (#8242; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-phospho-NF-κB p65 (#3031; Cell Signaling Technology), and rabbit anti-GAPDH antibodies (10494-1-AP; Proteintech, Rosemont, IL, USA). After overnight incubation at 4°C, each membrane was extensively washed with TBS-T, and further incubated with secondary antibody conjugated with horseradish peroxidase (HRP)-goat anti-mouse or anti-rabbit IgG antibody (SA00001-1 and SA00001-2, respectively; Proteintech). The secondary antibody was diluted 10,000-fold with 5% skim milk in TBS-T, and each membrane was incubated for 60 min at room temperature. After extensive washing with TBS-T, EzWestLumi plus (ATTO, Tokyo, Japan) was used as a substrate for HRP, and the signal was visualized and analyzed using the iBright Imaging System (Thermo Fisher Scientific).
Cell viability assayCell viability was determined using the Cell Count Kit-8 (Dojin Molecular Technologies) according to the manufacturer’s protocol. In brief, HeLa cells transfected with mock, RAGE-, and RAGEΔcyto-expressing vectors were selected by culturing them in DMEM containing 500 μg/mL hygromycin for a week, and seeded in a 96-well plate at a density of 1 × 104 cells/well. After 24 hr culture, the cells were washed twice with DMEM without FBS or hygromycin and treated with DHP-3 for 24 hr. After treatment, the cells were washed twice with phosphate-buffered saline and incubated in DMEM containing 10% CCK-8 solution for 2 hr in a CO2 incubator to generate the pigment. The absorbance at 450 nm was measured using a plate reader (SUNRISE Remote; FUJIFILM Wako Pure Chemical). The LC50 value, which was calculated with GraphPad Prism 5.04 software (GraphPad Software, La Jolla, CA, USA), is represented as the value ± standard error in Results and Discussion.
To validate the involvement of RAGE in DHP-3-induced cytotoxicity, we transfected RAGE or its deletion mutant RAGEΔcyto into HeLa cells using an episomal vector. The transient expression was confirmed by immunoblotting using whole cell lysates. A specific single band was detected in RAGE- and RAGEΔcyto-expressing HeLa cells, whereas no band was detected in mock-transfected cells (Fig. 2B). RAGE contains 404 residues, and its mass is 43 kDa. The apparent molecular size of RAGE is known to be shifted to 45–55 kDa by immunoblotting because there are two potential N-glycosylation sites, 25N and 81N, in its sequence (Azhary et al., 2020; Bertheloot et al., 2016; Xue et al., 2014). The size of the single band in RAGE-expressing cells was estimated to be 53 kDa, which was almost the same as the reported molecular size of RAGE. The band size in RAGEΔcyto-expressing cells was lower than that in RAGE-expressing cells (Fig. 2B). The difference in size (5 kDa) was consistent with the size of the lacking cytoplasmic domain; therefore, these results indicate that the subcloning and transfection were successful. Therefore, we examined hygromycin concentrations to select HeLa cells that were positively transfected. The transient expression levels of RAGE were apparently low (Fig. 2C, Lane 2). In addition, RAGE expression was not detected in the absence of hygromycin (hygromycin = 0 µg/mL), although RAGE expression was clearly observed at the other hygromycin concentrations (250, 500, 750, 1,000 µg/mL) (Fig. 2C). These results suggested that the transfection efficiency of the constructed episomal vector in HeLa cells was relatively low, but RAGE-expressing cells could be concentrated following culture for one week in the presence of 500 μg/mL hygromycin.
Next, to evaluate the involvement of RAGE in DHP-3-induced cytotoxicity, we examined the viability of RAGE- and RAGEΔcyto-expressing HeLa cells. Before the assay, the expression of RAGE and RAGEΔcyto in the cells was confirmed by immunoblotting (Fig. 3A). In addition, RAGE is known to induce inflammation and cytotoxicity mainly through the NF-κB pathway (Bierhaus et al., 2005; Huttunen et al., 1999). Thus, HeLa cells are widely used to analyze the effects of RAGE because they retain the NF-κB pathway (Jia et al., 2020; Ichiki et al., 2016). In this study, the overexpression of RAGE and RAGEΔcyto had minimal impact on NF-κB p65 and phospho-NF-κB p65 in the absence of the RAGE ligand (Fig. 3A). Thus, we excluded the possibility of ligand-independent effects induced by RAGE and RAGEΔcyto overexpression on our experimental system. The concentrations of DHP-3 were determined according to the results of previous studies (Esaki et al., 2020; Ishida et al., 2014). Fig. 3B shows the results of the cell viability assay following treatment of mock, RAGE, and RAGEΔcyto transfected cells with DHP-3. A dose-dependent decrease in cell viability was observed in the mock-transfected HeLa cells (Fig. 3B). Our previous study reported that the LC50 value of DHP-3 on HepG2 cells was 155.4 µM (Esaki et al., 2020). Compared to that, HeLa cells seemed to be less sensitive to DHP-3 because the LC50 value of DHP-3 in mock-transfected HeLa cells was apparently higher than that of HepG2 cells (LC50 of mock-transfected cells = 898.8 ± 129.5 µM). In contrast, although dose-dependent decrease in cell viability was also observed in RAGE- and RAGEΔcyto-expressing HeLa cells, significant differences in the LC50 value were not detected among the groups (RAGE-expressing cells, LC50 = 823.4 ± 100.3 µM; RAGEΔcyto-expressing cells, LC50 = 1014 ± 209.4 μM). Although RAGEΔcyto retains its ligand-binding capacity, it cannot transduce any cellular signaling (Huttunen et al., 1999). Thus, our results suggest that signal transduction starting from RAGE activation has little contribution to DHP-3-induced cytotoxicity.
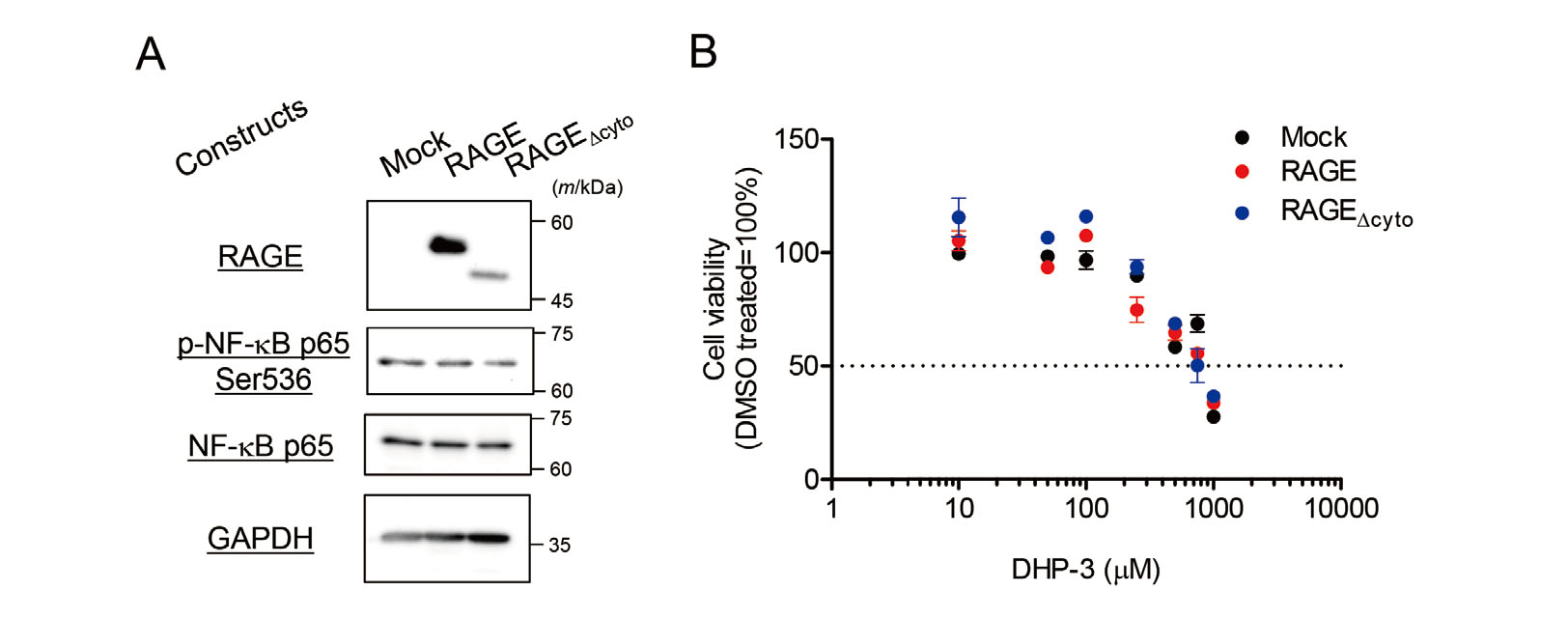
DHP-3-induced cytotoxicity in HeLa cells that express RAGE and RAGEΔcyto. (A) Detection of RAGE, RAGEΔcyto, and NF-κB p65 in HeLa cells cultured in the presence of 500 μg/mL hygromycin for a week. Cell lysates (20 μg) were electrophoresed and analyzed by immunoblotting. GAPDH was also detected as a loading control. The same batch of cells was utilized in the later cytotoxicity assay. (B) Cell viability of RAGE- and RAGEΔcyto-expressing HeLa cells following treatment with DHP-3. Cell viability was determined with Cell Counting Kit-8 (Dojin Molecular Technologies). Each plot represents the mean ± S.E. of a triplicate assay. This experiment was repeated twice, and a representative one is shown.
RAGE recognizes many molecules as ligands, including AGEs. However, it is also known that not all glycation products increase RAGE signaling (Sparvero et al., 2009). In addition, it has been reported that other cell surface receptors such as the AGE-receptor complex (OST-48/80K-H/galectin-3 complex) (Li et al., 1996; Vlassara et al., 1995) contribute to the recognition of AGEs. Except for RAGE, there is little information regarding the specificity of ligand recognition and the biological effects of these AGEs-recognizing receptors. It remains unknown whether DHP-3 binds to receptors other than RAGE; however, there is a possibility that DHP-3 elicits its cytotoxic effect by binding to AGEs-recognizing receptors other than RAGE. Additionally, it is likely that DHP-3 directly permeates through the cell membrane and produces cytotoxic effects via its chemical characteristics, such as its binding to glutathione (Takechi et al., 2015) and generation of radicals (Yamaguchi et al., 2012). Further studies are necessary to elucidate the molecular mechanisms underlying DHPs-mediated cellular effects.
This work was supported in part by JSPS KAKENHI (Grant-in-Aid for Early-Career Scientists) Grant Number 20K16045 (Recipient YM). The authors thank Ms. Natsumi Ito and Ms. Mayuko Oike for helping us in a prat of experiment.
Conflict of interestThe authors declare that there is no conflict of interest.